Covid Vaccine Janssen Approval | Janssen Covid 19 Vaccine Approved For Continued Use In Us
The Janssen COVID-19 Vaccine is an unapproved vaccine that may prevent COVID-19. In an ongoing clinical trial the Janssen COVID -19 Vaccine has been shown to prevent COVID -19 following a single dose.

Johnson Johnson Withdraws Covid 19 Vaccine Approval Proposal In India Coronavirus Outbreak News
94 effective at preventing the COVID-19 virus with symptoms.

Covid vaccine janssen approval. 28 May 2021 The COVID-19 Vaccine Janssen has today been given regulatory approval by the Medicines and Healthcare products Regulatory Agency MHRA. FDA approves Comirnaty COVID-19 Vaccine mRNA which was previously known as Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID-19 disease in individuals 16 years of. Janssen COVID-19 Vaccine Johnson Johnson.
The Janssen COVID-19 Vaccine has not been approved or licensed by the US. COVID-19 vaccination is recommended for all people aged 12 years and older including people who are pregnant breastfeeding trying to get pregnant now or might become pregnant in the future. Monday September 6 2021.
COVID-19 vaccines Pfizer-BioNTech Moderna AstraZeneca COVISHIELD Janssen not available in BC Type of Vaccine mRNA mRNA Non-replicating viral vector Non-replicating viral vector Date authorized for use in Canada Dec 9 2020 Dec 23 2020 Feb 26 2021 Mar 5 2021 Age indication years of age 12 12 18 18 Dose 03 mL 05. August 23 2021. The EUA for the Janssen COVID-19 Vaccine was issued to Janssen Biotech Inc a Janssen Pharmaceutical Company of Johnson Johnson.
Six of those have been approved for emergency or full use by at least one WHO -recognized stringent regulatory authority OxfordAstraZeneca Pfizer-BioNTech Sinopharm-BBIBP Moderna Sinovac and Janssen. The Summary of Product Characteristics is a description of a. On 18 February 2021 the vaccine received emergency authorization in South Africa.
In an ongoing clinical trial 21895. On 13 April 2021 South Africa suspended its rollout of the vaccine. Information about the COVID-19 Vaccine Janssen approved by the MHRA on 28 May 2021.
The Janssen COVID-19 Vaccine is an unapproved vaccine. However COVID-19 constantly evolves. Effective April 23 2021 CDC and FDA recommend that use of the Janssen COVID-19 Vaccine resume in the United States.
WHAT ARE THE RISKS OF THE JANSSEN COVID-19 VACCINE. Pregnant and recently pregnant people are more likely to get severely ill with COVID-19 compared with non-pregnant people. Effective April 23 2021 CDC and FDA recommend that use of the Johnson Johnsons Janssen JJJanssen COVID-19 Vaccine resume in the United States.
The authorization will be effective until the declaration that. The COVID-19 Vaccine Janssen has today been given regulatory approval by the Medicines and Healthcare products Regulatory Agency MHRA. 6 12 and 24 months after 2-dose primary regimen.
Food and Drug Administration issued an. These side effects usually start within a day or two of getting the vaccine. People had the most protection 2 weeks after getting vaccinated.
National regulatory authorities have granted emergency use authorizations for twenty-two COVID-19 vaccines. B An open-label extension will allow participants who initially received placebo to receive a single dose of Ad26COV2S vaccine. Janssen COVID-19 Vaccine Fact Sheets and FAQs Fact Sheet Translations On February 27 2021 the US.
The JJJanssen COVID-19 Vaccine was 663 effective in clinical trials efficacy at preventing laboratory-confirmed COVID-19 infection in people who received the vaccine and had no evidence of being previously infected. FDA approves Comirnaty COVID-19 Vaccine mRNA. The program resumed on.
115 rows August 23 2021. The duration of protection against COVID-19 is currently unknown. The World Health Organization issued an EUL for the Janssen COVID-19 vaccine Ad26COV2S vaccine on 12 March 2021.
Vaccinesgov helps you find clinics pharmacies and other locations that offer COVID-19 vaccines in the United States. This is the fourth COVID-19 vaccine to be.

Eu Countries Ready To Start Using J J Shot As Deliveries Resume Reuters
Janssen Covid 19 Vaccine Authorised For Use In Eu

Covid Vaccine Eu Regulators Approve Johnson Johnson Shot News Dw 11 03 2021
Eu Commission Approves Johnson Johnson S One Shot Covid 19 Vaccine
/cloudfront-us-east-2.images.arcpublishing.com/reuters/VKXFTR4CXFOTHIAWAZIF4NB6HA.jpg)
Germany Sets No Limits On Use Of J J Covid 19 Shot Reuters
Janssen Covid 19 Vaccine Approved For Continued Use In Us

Covid 19 Johnson Johnson Vaccine Pause Is Another Hurdle For Europe The New York Times

Covid Vaccine Ema Says J J Shot Can Be Rolled Out Across Eu

Coronavirus Digest Johnson Johnson Applies For Emergency Approval In Us News Dw 05 02 2021

Single Dose Janssen Covid 19 Vaccine Receives Regulatory Approval From The Mhra Technology Networks

Ema Issues Warning Over Johnson Johnson Vaccine Stops Short Of Ruling Against Use News Dw 20 04 2021
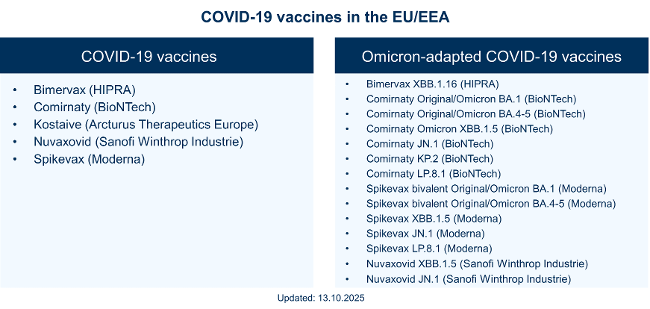
Paul Ehrlich Institut Dossier Coronavirus Sars Cov 2 And Covid 19coronavirus And Covid 19

Janssen Covid 19 Vaccine Expiry Checker

France Approves J J S Janssen Covid 19 Vaccine Reuters
Paul Ehrlich Institut News Covid 19 Vaccine Janssen Possible Relation To Very Rare Cases Of Unusual Blood Clots In Combination With A Reduced Platelet Count

European Regulator Gives Approval To Johnson Johnson One Shot Vaccine Voice Of America English

Coronavirus Georgia Pauses J J Vaccine At One Site Following Adverse Reactions As It Happened Financial Times
Janssen Presents One Shot Covid 19 Vaccine Data European Biotechnology

J J Applies To Ema For Covid Vaccine Approval Chemanager


Post a Comment
Post a Comment